No products in the cart.
MED 126 PRO-ACTIVE
Roll over image to zoom in
4,079.55USD
MED 126 PRO-ACTIVE
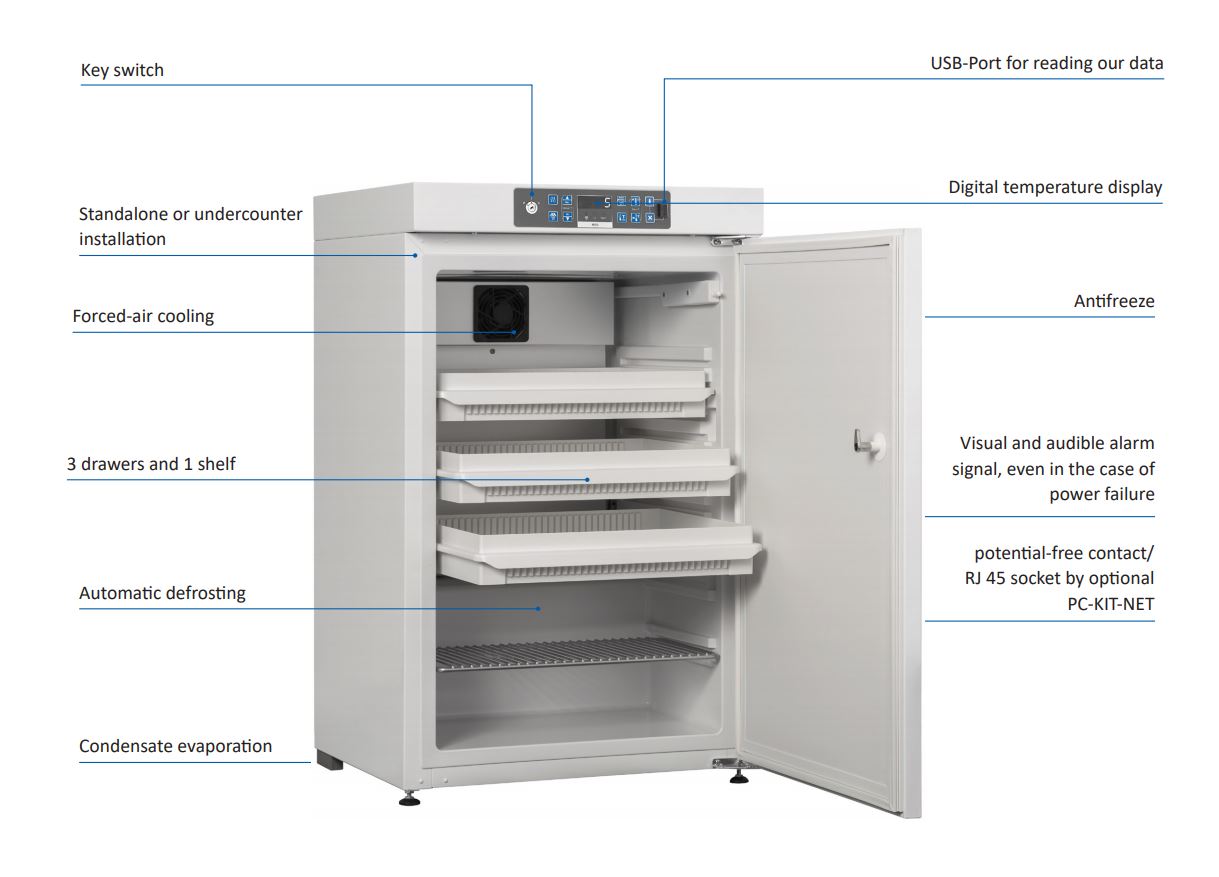
• PRO-ACTIVE-Control: Permanent, proactive monitoring of
the performance data and alerts in the case of deviations
– so that you can take countermeasures in good time
before a fault threatens your chilled goods; world´s most
accurate temperature control in refrigerated areas thanks
to two standard PT-1000 sensors
• External housing made from galvanised sheet steel
(rustproof), with white, anti-scratch powder coating.
Length of plug cable: approx. 2.8 m.
• Adjustable feet positioned at the front to compensate
uneven floors
• Interior made from impact-resistant, white plastic with
moulded-in shelves.
• nterior consists of three drawers with stops and a plasticcoated
shelf. There is one adjustable length divider and
six adjustable cross dividers per drawer. Storage surface
per drawer = 0.13 m².
• Extra-thick 50-mm insulation, made from high-quality,
compression-moulded and environmentally friendly material.
Energy saving.
• Door with easy-to-replace plastic magnetic seal frame,
lockable.
• Door hinge on the right-hand side (by default, see illustration), or
the left-hand side, can be retrofitted.
• Forced air cooling, switches off automatically when you
open the door, ensures a uniform temperature and
reduces temperature reductions to a minimum.
• Automatic defrosting with time limit and temperature
monitoring.
• Condensate evaporation in the refrigerating machine
interior.
• Electronic temperature control with membrane keyboard
and digital temperature display in the interior.
• Warning functions: visual and audible alarm signal, even
in the case of temperature deviations and power failure.
In the case of power failure, the monitoring unit remains
in operation for approx. 30 hours on battery power. Door
open alarm after 60 seconds.
• Alarms can be forwarded using a potential-free contact
(e.g., to a mobile phone with optional GSM-Module or to
a control centre).
• Antifreeze against sub-zero temperatures.
• Statically ventilated refrigerating machine, hermetically
sealed, energy saving, low noise, easy to service.
Optional equipment :
• Glass door.
• Tabletop (exterior dimensions: 84 cm in height).
• Decorative door frame for attaching the customer‘s
decorative plates (exterior dimensions: 55 cm in width).
• Door fitting.
• Equipped with castors.
• Top drawer can be locked.
• Additional length and cross dividers.
• Shelf w x d = 43.8 x 33 cm instead of drawer.
• Digital tempeMeasured values based onature display:
optional for external installation into furniture front.
• Condensate container can be emptied manually if no
condensation is required (e.g., in an operating theatre).
• Refrigerating machine fan if static ventilation is not sufficient
after installation, or if 60 Hz operation is intended.
• GSM-Module for alarm text message transmission
(e.g., to a mobile phone).
• Temperature documentation:
– PC-KIT-NET
– 4-pin PT 100 or PT 1000 temperature sensor, class
1/3 B, including built-in reference body (measurement
bottle or refrigerating block).
– Mechanical pen-recording thermometer with two
waxed paper strips.
Additional information
| Weight | 57 kg |
|---|---|
| Dimensions | 54 × 54 × 81 cm |
| Capacity | 120 L |
| Temperature setting | approx. +2 °C to +15 °C |
| Voltage | 220 – 240 V, 50 Hz |
| Power consumption | 105 watts |
| Normal consumption | 0.58 kWh/24 h |
| Admissible ambient temperature | from +10 °C to +38 °C |
| Heat emission (max.) | 183 watts |
| Interior dimensions | w x d x h = 44 x 42 x 67 cm |
| Exterior dimensions with door open at 90° | w x d = 54 x 105.5 cm |
| Shelf size | 43.8 x 24 cm |
| Drawer inner dimensions | w x d x h = 40.8 x 32 x 5.6 cm |
| Max. load drawer/shelf | 13 kg/25 kg |
| Model | MED 126 PRO-ACTIVE |